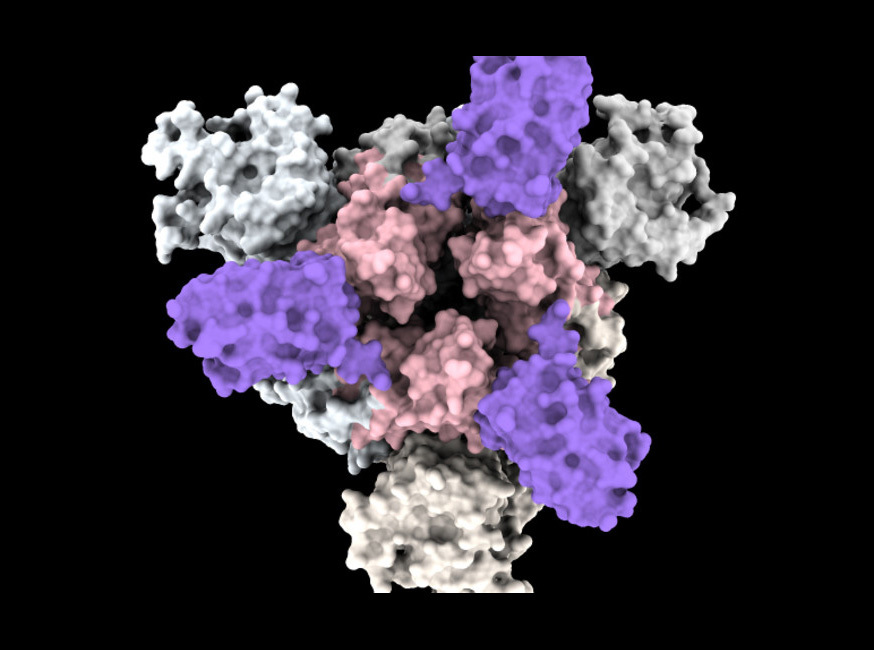
Nano-antibodies [in purple] settle on the spike of the corona virus.
(photo credit: Photo from Dr. Dina Schneidemann's laboratory)
A new drug platform for speedily generating anti-viral drugs that target proteins common to all viruses has been developed by a startup named ViroBlock established by Hebrew University of Jerusalem (HU) researchers, they announced on Wednesday.
About a million kinds of viruses infect vertebrates, and a third of them invade mammals; of these, there are 219 virus species known to be able to infect humans. The first to be discovered was yellow fever in 1901; three to four new species are still being discovered every year.
Though these microscopic “genetic parasites” – which contain DNA or RNA and need to invade a host so as in order to multiply – are very varied, viruses still have many things in common.
Not strictly defined as living organisms, they have exacted a tremendous toll on human wellbeing through the ages.
Notorious examples include smallpox, one of the deadliest diseases in human history that killed 200 million people in the 20th century alone; the flu virus, which kills some 500,000 people each year and during the Spanish flu pandemic killed 50 million to 100 million people worldwide; and HIV/AIDS, which has killed 30 million people since its emergence in the 1980s.
Viruses continue to emerge – in Africa, the Marburg and Ebola viruses are particularly deadly, while the global COVID-19 pandemic is a reminder of mankind’s precarious vulnerability.
ViroBlock's new drug
Now, Hu's ViroBlock has developed a new drug platform for speedily generating anti-viral drugs that target proteins common to all viruses.
The new drug targets common viruses and could treat current and future COVID-19 variants, influenza, Zika, West Nile and hepatitis B.
What did the researchers say?
“Currently, there are no efficient, validated platforms for rapidly generating anti-viral drugs,” said ViroBlock CEO and founder Prof. Isaiah (Shy) Arkin, who teaches and researches biological chemistry at HU’s Alexander Silberman Institute of Life Sciences.
“Scientists must develop new agents and a customized approach to target every new virus – without the ability to predict how that virus will develop resistance," he said. "Launched in 2020 by Yissum, HU’s technology transfer company, ViroBlock is working on a promising drug candidate for COVID-19 using an approach that can be duplicated with most other important viruses.”
How does it work?
ViroBlock developed an approach to map a pathogen’s resistance options against its inhibitor before any clinical use. Using this approach to influenza, company scientists rapidly identified all clinically known resistant mutations in a completely unbiased and risk-free manner.

Prof. Roger Kornberg
Prof. Roger David Kornberg, an American expert in structural biology at the Stanford University School of Medicine – who received a Nobel Prize in Chemistry in 2006 for his studies of the process by which genetic information from DNA is copied to RNA – is head of the startup’s scientific advisory board.
According to a new study conducted by pharma research company Evotec, ViroBlock’s new technology platform proved its potential to rapidly provide solutions for treating existing and emerging viral threats.
ViroBlock plans to target and exploit a particular vulnerability in the viruses that cause these diseases — ion channels. These are critical components of the virus that enable it to regulate its salinity and acidity.
As a result, inhibiting such channels is a promising and proven route to curb the virus’s ability to infect cells.
The startup developed rapid screening approaches that can identify inhibitors from thousands of chemicals in a matter of weeks and can accurately predict the virus's resistance options against any inhibitor.
All cells are surrounded by membranes, whether from animals, plants or bacteria. However, as impermeable barriers, membranes require transport systems, such as channels, to regulate salts’ passage through them. Therefore, it is no surprise that all living organisms possess many channels that allow their cells to control the salinity and acidity of their interior and surroundings.
The study showed that channel blockers that disrupt the movement of calcium in the cells could protect them from viral-induced death while dramatically lowering the number of viruses reproduced.
ViroBlock's antiviral drug candidates inhibit the E (envelope) protein and the 3a protein. The E protein is an ion channel, a type of protein family expressed by virtually all living cells that, because of its structure, has served as a frequent target for pharmaceutical point interventions.
For example, calcium channel blockers are routinely used to combat hypertension by decreasing blood pressure, and sodium and potassium channel blockers are often used to treat irregular heartbeat.
Another example: While the spike proteins of SARS-CoV-2 (COVID-19) and SARS-CoV-1 (the virus that appeared in 2003) are only about 75% identical, their E proteins are roughly 95% alike. This means the ViroBlock drugs would likely remain effective even when the virus mutates.
“With our proprietary technology, ViroBlock can identify targets in a new viral threat (or variant), develop inhibitors against it and determine the resistance potential of the virus against the new drug, all at an unprecedented pace,” Arkin said.
The next phase of clinical trials will test the efficacy of this anti-viral approach for humans. The company also has drugs in the pipeline produced by the platform currently being tested that could be effective against other viruses.
Story Source: THE JERUSALEM POST. Original written by JUDY SIEGEL-ITZKOVICH. “New platform will speed-develop drugs to combat future COVID-19 variants”.
https://www.jpost.com/health-and-wellness/coronavirus/article-711343
Note: Content may be edited for style and length.





